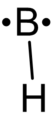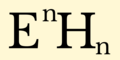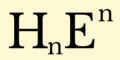Category:Hydrides
Jump to navigation
Jump to search
English: Hydride is the name given to the negative ion of hydrogen, H−. Although this ion does not exist except in extraordinary conditions, the term hydride is widely applied to describe compounds of hydrogen with other elements, particularly those of groups 1–16. The variety of compounds formed by hydrogen is vast, arguably greater than that of any other element. Various metal hydrides are currently being studied for use as a means of hydrogen storage in fuel cell-powered electric cars and batteries. They also have important uses in organic chemistry as powerful reducing agents, and many promising uses in hydrogen economy.
Every element of the periodic table (except some noble gases) forms one or more hydrides. These compounds may be classified into three main types by the predominant nature of their bonding:
- Saline hydrides, which have significant ionic character,
- Covalent hydrides, which include the hydrocarbons and many other compounds, and
- Interstitial hydrides, which may be described as having metallic bonding.
any chemical compound having a hydrogen atom bonded to a more electropositive element or groups | |||||
| Upload media | |||||
| Instance of |
| ||||
|---|---|---|---|---|---|
| Subclass of | |||||
| Has part(s) | |||||
| |||||
Subcategories
This category has the following 11 subcategories, out of 11 total.
Media in category "Hydrides"
The following 39 files are in this category, out of 39 total.
-
Acyclic Germylene Hydride.jpg 708 × 134; 15 KB
-
Arsinogermane.png 514 × 114; 6 KB
-
Benzimidazoline hydride.png 717 × 503; 10 KB
-
Boron monohydride.png 112 × 258; 3 KB
-
Cadmium dihydride.png 348 × 260; 4 KB
-
CO2 Reduction Mechanism.jpg 558 × 324; 26 KB
-
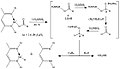
Direct Germylene Hydride Synthesis.jpg 655 × 229; 24 KB
-
ELF Ge2H.png 1,200 × 579; 72 KB
-
EuH2 in PbCl2.jpg 5,064 × 3,920; 674 KB
-
Ge2H Synthesis.jpg 732 × 256; 31 KB
-
GeH Molecular Graphs.png 787 × 777; 137 KB
-
GeH Resonance.jpg 948 × 248; 37 KB
-
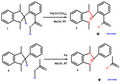
Germylene Hydride Reactions.jpg 970 × 907; 131 KB
-
Germylene Hydride Synthesis from Adduct.jpg 826 × 229; 33 KB
-
Germyliumylidene Hydride Synthesis.jpg 964 × 159; 26 KB
-
Group6ABP.jpg 568 × 344; 24 KB
-
Group6AMP.jpg 567 × 338; 21 KB
-
Hafnium hydride.jpg 2,048 × 1,536; 1.82 MB
-
Hydride ED Mechnism.png 468 × 81; 8 KB
-
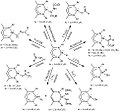
Hydride formula (I-XV).png 2,000 × 1,000; 41 KB
-
Hydride formula (XVI-XVIII).png 2,000 × 1,000; 41 KB
-
Hydriididenimetused.jpg 565 × 377; 57 KB
-
Hydroboration Germylene Hydride.jpg 1,007 × 481; 47 KB
-
KMgH3 + Eu2+.gif 1,897 × 1,697; 275 KB
-
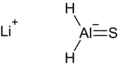
LiAlH2S.png 694 × 380; 8 KB
-
Lithium barium hydride + Eu2+.gif 985 × 531; 72 KB
-
Lithium gallium hydride.png 522 × 454; 6 KB
-
Lithium indium hydride.png 522 × 454; 5 KB
-
Lithium strontium hydride + Eu2+.gif 985 × 526; 71 KB
-
Lithium thallium hydride.png 522 × 454; 4 KB
-
Magnesium nickel hydride.png 506 × 480; 12 KB
-
Mg---FeH6.svg 132 × 124; 7 KB
-
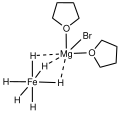
Mg4Br4(thf)8FeH6.svg 175 × 70; 5 KB
-
MH2.png 539 × 296; 3 KB
-
Olis dsipergeeritud naatriumhüdriid.jpg 1,840 × 3,264; 1.44 MB
-
Pentaszilan vonalas-atomcsoportos keplete.jpg 201 × 18; 2 KB
-
SbH3 burning in oxygen.jpg 1,536 × 2,048; 427 KB
-
SbH3 burning in the air.jpg 1,536 × 2,048; 379 KB
-
Zinc hydride.png 366 × 228; 3 KB