File:Borax-unit-cell-3D-balls.png

Original file (1,100 × 831 pixels, file size: 512 KB, MIME type: image/png)
Captions
Captions
| DescriptionBorax-unit-cell-3D-balls.png |
Ball-and-stick model of the unit cell of borax ("sodium tetraborate decahydrate"), Na2B4O7·10H2O, better described as Na2[B4O5(OH)4]·8H2O. Boron is pink, oxygen is red, hydrogen is white, and sodium is lilac. The structure includes 8 water molecules for each tetraborate anion (not shown, for clarity). Neutron diffraction data from H. A. Levy and G. C. Lisensky (December 1978). "Crystal structures of sodium sulfate decahydrate (Glauber's salt) and sodium tetraborate decahydrate (borax). Redetermination by neutron diffraction". Acta. Cryst. B 34 (12): 3502-3510. DOI:10.1107/S0567740878011504. The anion's formula is [ B4O5(OH)42- ]. A refined structure was published in G. J. Gainsford, T. Kemmitt and C. Higham (2008): "Redetermination of the borax structure from laboratory X-ray data at 145 K". Acta Crystallographica Series E (Inorganic Compounds), volume E64, pages i24-i25. doi:10.1107/S1600536808010441 According to this source, each sodium ion is surrounded by 6 water molecules, 4 of which are shared with 4 other sodium cations, forming zigzag chains. |
|||
| Date | ||||
| Source | Own work | |||
| Author | Ben Mills | |||
| Permission (Reusing this file) |
|
File history
Click on a date/time to view the file as it appeared at that time.
| Date/Time | Thumbnail | Dimensions | User | Comment | |
|---|---|---|---|---|---|
| current | 22:54, 1 May 2008 | 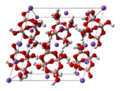 | 1,100 × 831 (512 KB) | Benjah-bmm27 (talk | contribs) | {{Information |Description = Ball-and-stick model of the unit cell of borax ("sodium tetraborate decahydrate"), Na<sub>2</sub>B<sub>4</sub>O<sub>7</sub>·10H<sub>2</sub>O, better described as Na<sub>2</sub>[B<sub>4</sub>O<sub>5</sub>(OH)<sub>4</sub>]·8H< |
You cannot overwrite this file.
File usage on Commons
There are no pages that use this file.
File usage on other wikis
The following other wikis use this file:
- Usage on af.wikipedia.org
- Usage on ar.wikipedia.org
- Usage on bn.wikipedia.org
- Usage on de.wikipedia.org
- Usage on en.wikipedia.org
- Usage on eo.wikipedia.org
- Usage on fa.wikipedia.org
- Usage on fi.wikipedia.org
- Usage on fr.wikipedia.org
- Usage on hu.wikipedia.org
- Usage on id.wikipedia.org
- Usage on ja.wikipedia.org
- Usage on jv.wikipedia.org
- Usage on ka.wikipedia.org
- Usage on lt.wikipedia.org
- Usage on lv.wikipedia.org
- Usage on ms.wikipedia.org
- Usage on nl.wikipedia.org
- Usage on pl.wikipedia.org
- Usage on pt.wikipedia.org
- Usage on ru.wikipedia.org
- Usage on sco.wikipedia.org
- Usage on sh.wikipedia.org
- Usage on sr.wikipedia.org
- Usage on ta.wikipedia.org
- Usage on te.wikipedia.org
- Usage on ur.wikipedia.org